|
The chemical and biophysical properties of macromolecules are at the core of most biological phenotypes. Developing a molecular-level understanding of key macromolecules is therefore critical to both understanding and engineering more complex biological systems. When possible, we work with collaborators to develop a better biophysical understanding of macromolecules that may be of further scientific and/or biotechnological interest. To date, studies have focused primarily on the haloarchaeal opsins and haloarchaeal lipid systems. |
Structure and Biophysics of Microbial Opsins
|
| Collaboration with Prof. Yong Duan (UCD-BME), Ting Wang, and Robert Glaeser (LBNL) |
| The archaeal opsins serve important roles as transmembrane ion pumps, ion channels, and sensory proteins. Bacteriorhodopsin (bR), the heptahelical light-driven proton pump of the archaeon Halobacterium salinarum, is one of the best-studied archaeal opsins (and membrane proteins generally) from which we have derived fundamental knowledge about the ubiquitous process of proton transport. With collaborators Drs. Ting Wang, Yong Duan, and Robert Glaeser, we have investigated the structure and molecular dynamics of a key structurally labile mutant of bacteriorhodopsin. In so doing, we have shed light into the molecular mechanism underlying proton uptake. On-going studies are investigating other functional features of ion translocation. We expect that such studies will lead to directed engineering microbial opsins for biotechnological application, including applications related to optogenetics.
|
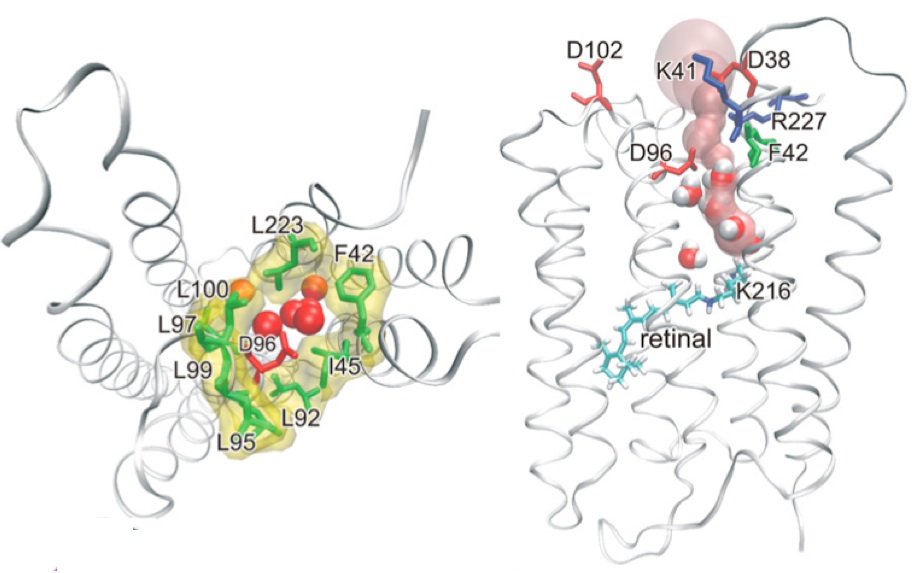 - X-ray crystal structure of the D96A/F219L/F171C mutant of bacteriorhodopsin. Adapted from (Wang, T, Sessions A, Lunde C, Rouhani S, Glaeser R, Duan Y, & Facciotti MT. 2013. Deprotonation of D96 in Bacteriorhodopsin Opens the Proton Uptake Pathway Structure 21, 290-7.)
|
Haloarchaeal Membrane Biophysics
|
| Collaboration with Prof. Atul Parikh (UCD-BME) and graduate student Sean Gilmore |
| Archaeal membranes are composed of ether-linked isoprenoid-derived lipids that are distinctly different from the ester-linked aliphatic lipids commonly found in bacteria or eukaryotes. These membranes have unique biochemical and biophysical properties that are thought to help provide a first line of defense against environmental assaults these organisms encounter in the extreme environments inhabited by many archaeal species. We are working with collaborators Prof. Atul Parikh and PhD student Sean Gilmore to characterize how the biophysical properties of lipids in Halobacterium salinarum NRC-1 , namely the interfacial compressibility and lateral organization (both influenced pronouncedly by cholesterol in mammalian cells) are influenced by squalene. Mr. Gilmore’s current data indicate that squalene has a role analogous to cholesterol in phospholipid bilayers, suggesting the possibility that it might play a crucial role modulating chain order, lipid packing, and domain formation in the membranes of archaea.
|
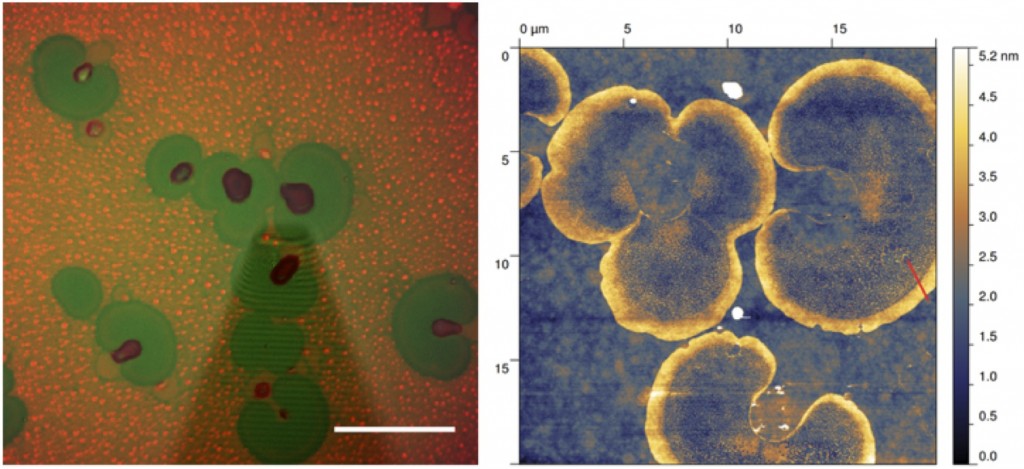 - Images of structured haloarchaeal lipid domains obtained by epifluorescence microscopy and atomic force microscopy (Sean Gilmore).
|